Industry Adopts Brake Fluid
Replacement Guidelines
By Bill Williams,
Technical Contributor There has been much controversy involving brake fluid over the years. Much of this controversy involves the issue of "when" and "why" to change the brake fluid. The controversy has increased in the last few years largely based on the following:
- The introduction of new brake fluid testing technology;
- Statements made by the "Big 3" domestic OEM manufacturers that the brake fluid in their vehicles lasts the life of the vehicle; and
- The fact that the last three stings involving the auto service industry involved some mention of brake fluid.
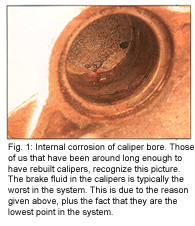 While it is generally accepted that brake fluid should be changed
periodically the justification for such a recommendation varies widely. Most
technicians have based much of their recommendations on the fact that DOT 3
brake fluid is hygroscopic. Hygroscopic means the brake fluid readily
absorbs water. Water is absorbed through all the rubber parts in the system,
including the brake hoses and seals, and every time the reservoir cap is
removed. It has long been thought that the moisture absorbed in the brake
fluid was the sole source for the internal corrosion that takes place in the
various brake parts.
I will be the first to admit I, like many others have blamed this
type of corrosion and sludge build-up entirely on the moisture content of
the brake fluid. This position has recently been proven incorrect. The
moisture content in the brake fluid is NOT the only cause of the corrosion
that takes place in a brake system; it is only a contributing factor.
While it is generally accepted that brake fluid should be changed
periodically the justification for such a recommendation varies widely. Most
technicians have based much of their recommendations on the fact that DOT 3
brake fluid is hygroscopic. Hygroscopic means the brake fluid readily
absorbs water. Water is absorbed through all the rubber parts in the system,
including the brake hoses and seals, and every time the reservoir cap is
removed. It has long been thought that the moisture absorbed in the brake
fluid was the sole source for the internal corrosion that takes place in the
various brake parts.
I will be the first to admit I, like many others have blamed this
type of corrosion and sludge build-up entirely on the moisture content of
the brake fluid. This position has recently been proven incorrect. The
moisture content in the brake fluid is NOT the only cause of the corrosion
that takes place in a brake system; it is only a contributing factor.
 It is not often that an entire industry's
position on something can be shown to be incorrect but it can happen. The
issue of brake fluid testing came under the scrutiny of the Maintenance
Services Task Force of the AMRA (Automotive Maintenance and Repair
Association) back in December of 2002. The task force was represented by
members from the auto service industry, equipment manufacturers, parts
manufacturers, education and the scientific community. Just recently, the
task force released their findings. This article will serve to explain the
process they went through and their ultimate conclusion.
The initial goal of the task force was to determine if
industry-accepted guidelines could be adopted in the area of brake fluid
flushing. Early on in the process, it was apparent to all those on the task
force that the opinions about why brake fluid should be flushed varied
widely and most of them had something to do with the moisture content of the
brake fluid and its boiling point. The task force's direction evolved from
the issue of fluid flushing to that of how to determine if a brake fluid
flush should be suggested or required.
It is not often that an entire industry's
position on something can be shown to be incorrect but it can happen. The
issue of brake fluid testing came under the scrutiny of the Maintenance
Services Task Force of the AMRA (Automotive Maintenance and Repair
Association) back in December of 2002. The task force was represented by
members from the auto service industry, equipment manufacturers, parts
manufacturers, education and the scientific community. Just recently, the
task force released their findings. This article will serve to explain the
process they went through and their ultimate conclusion.
The initial goal of the task force was to determine if
industry-accepted guidelines could be adopted in the area of brake fluid
flushing. Early on in the process, it was apparent to all those on the task
force that the opinions about why brake fluid should be flushed varied
widely and most of them had something to do with the moisture content of the
brake fluid and its boiling point. The task force's direction evolved from
the issue of fluid flushing to that of how to determine if a brake fluid
flush should be suggested or required.
 You might be saying to yourself: "Why would
you need a task force to determine something so simple?"
I, like many others am of the opinion that brake fluid represents the
most neglected fluid in an automobile. So what's the big deal? The big deal
is that the reason most shops give a customer for the reason why they should
have a brake fluid flush has no basis in fact.
The most common explanation given to a customer about why they should
have their brake fluid flushed is based on the fluid's color.
You might be saying to yourself: "Why would
you need a task force to determine something so simple?"
I, like many others am of the opinion that brake fluid represents the
most neglected fluid in an automobile. So what's the big deal? The big deal
is that the reason most shops give a customer for the reason why they should
have a brake fluid flush has no basis in fact.
The most common explanation given to a customer about why they should
have their brake fluid flushed is based on the fluid's color.
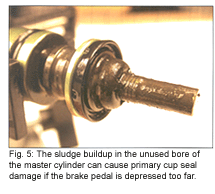 If you watch some of the recent sting videos
that have taken place on both a local and national level you will here
things like, "Your brake fluid is dirty and needs to be changed". This was
one of the first issues the task force dealt with. One of the things that
must be understood about the objective of the task force is that if they
were to make any recommendations on the issue of brake fluid flushing, they
must be based on fact, not commonly accepted opinion.
A number of independent sources pointed out that the color of brake
fluid cannot be used as an indicator of its condition. One brake fluid
manufacturer went on to explain that some new brake fluids have additives
that will cause the brake fluid to change color when exposed to brake rubber
parts. Another source provided data showing that brake fluid appearing
almost pitch black can pass all available tests while other fluid samples
appearing to be "good" could fail those same tests. So, one thing that
became obvious early on was our industry has to stop recommending brake
fluid flushes on the color of the brake fluid. If we don't stop, we will
continue to end up on the evening news.
If you watch some of the recent sting videos
that have taken place on both a local and national level you will here
things like, "Your brake fluid is dirty and needs to be changed". This was
one of the first issues the task force dealt with. One of the things that
must be understood about the objective of the task force is that if they
were to make any recommendations on the issue of brake fluid flushing, they
must be based on fact, not commonly accepted opinion.
A number of independent sources pointed out that the color of brake
fluid cannot be used as an indicator of its condition. One brake fluid
manufacturer went on to explain that some new brake fluids have additives
that will cause the brake fluid to change color when exposed to brake rubber
parts. Another source provided data showing that brake fluid appearing
almost pitch black can pass all available tests while other fluid samples
appearing to be "good" could fail those same tests. So, one thing that
became obvious early on was our industry has to stop recommending brake
fluid flushes on the color of the brake fluid. If we don't stop, we will
continue to end up on the evening news.
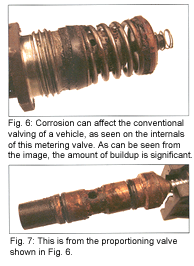 As the task force gathered more information
on the topic, it became clear that most of the literature available put the
focus on the moisture content and boiling point of the brake fluid as the
leading concerns. It was also clear that the majority of the current test
techniques available at the shop level center on providing a measure of the
moisture content, or boiling point of the brake fluid.
These techniques include strip testing, boiling point analysis,
refractometers and conductivity meters. Testing that provides an indication
of the moisture content or boiling point of the brake fluid is usually using
the DOT minimum wet boiling point of the brake fluid as a base line
reference. As it turns out, the minimum wet boiling point is not meant to be
used as an "in-use" specification. This paragraph excerpt from task force's
conclusion helps explain this:
As the task force gathered more information
on the topic, it became clear that most of the literature available put the
focus on the moisture content and boiling point of the brake fluid as the
leading concerns. It was also clear that the majority of the current test
techniques available at the shop level center on providing a measure of the
moisture content, or boiling point of the brake fluid.
These techniques include strip testing, boiling point analysis,
refractometers and conductivity meters. Testing that provides an indication
of the moisture content or boiling point of the brake fluid is usually using
the DOT minimum wet boiling point of the brake fluid as a base line
reference. As it turns out, the minimum wet boiling point is not meant to be
used as an "in-use" specification. This paragraph excerpt from task force's
conclusion helps explain this:
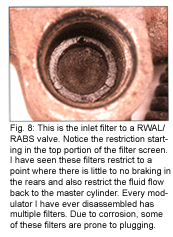 "Motor vehicle brake fluids are hygroscopic
and absorb moisture when exposed to the atmosphere and in service. Water
contamination from any source, including mechanical or accidental additions
of free water, will appreciably lower the original boiling point of the
brake fluid, and increase its viscosity at low ambient temperatures. Water
contamination may cause corrosion of brake cylinder bores and pistons, and
may seriously affect the braking efficiency and safety of the brake
actuating system.
Source: SAE international standard (J1707, Revised January 2002).
While moisture is definitely an issue with brake fluids, no
consistent and accurate measurement identifying the percentage of moisture
that is detrimental to brake fluid performance has been found in the
literature. In addition, no specification exists for an in-use brake fluid
boiling point that can be identified by a testing procedure and therefore no
recommendation for replacement based on moisture content can be made with
confidence."
In summary, while the moisture content of the brake fluid remains a
concern, no accurate test is available to determine when it should be
replaced based on moisture content.
There is another reason to take the focus off of the moisture content
of the brake fluid and place it where it belongs. There have been
significant advances in the technology used to make the rubber that is used
in brake hoses, square cut seals and wheel cylinder cup seals.
"Motor vehicle brake fluids are hygroscopic
and absorb moisture when exposed to the atmosphere and in service. Water
contamination from any source, including mechanical or accidental additions
of free water, will appreciably lower the original boiling point of the
brake fluid, and increase its viscosity at low ambient temperatures. Water
contamination may cause corrosion of brake cylinder bores and pistons, and
may seriously affect the braking efficiency and safety of the brake
actuating system.
Source: SAE international standard (J1707, Revised January 2002).
While moisture is definitely an issue with brake fluids, no
consistent and accurate measurement identifying the percentage of moisture
that is detrimental to brake fluid performance has been found in the
literature. In addition, no specification exists for an in-use brake fluid
boiling point that can be identified by a testing procedure and therefore no
recommendation for replacement based on moisture content can be made with
confidence."
In summary, while the moisture content of the brake fluid remains a
concern, no accurate test is available to determine when it should be
replaced based on moisture content.
There is another reason to take the focus off of the moisture content
of the brake fluid and place it where it belongs. There have been
significant advances in the technology used to make the rubber that is used
in brake hoses, square cut seals and wheel cylinder cup seals.
 This technology has made the rubber much
more resistant to moisture intrusion. The increased use of transparent
reservoirs has reduced the need to remove the cap to check the fluid level.
As a result of these changes, less moisture enters the brake system.
One of the task force members involved with the OEMs found out one of
the OEMs did a study using test fleets comprised of thousands of vehicles
from various locations that were approximately 8 years old. They tested the
brake fluid for moisture and discovered an average of less than one percent
over the entire fleet.
They said improved master cylinder reservoir design, brake fluid and
brake hose materials significantly reduced moisture related problems with
brake fluid. They did however find evidence of corrosion.
This last statement is the key to understanding the new twist being
put on the entire topic of brake fluid testing. Corrosion was found without
significant amounts of moisture in the system. To explain this we have to
take a step back and discuss how one of the purposes of brake fluid is to
prevent corrosion.
This technology has made the rubber much
more resistant to moisture intrusion. The increased use of transparent
reservoirs has reduced the need to remove the cap to check the fluid level.
As a result of these changes, less moisture enters the brake system.
One of the task force members involved with the OEMs found out one of
the OEMs did a study using test fleets comprised of thousands of vehicles
from various locations that were approximately 8 years old. They tested the
brake fluid for moisture and discovered an average of less than one percent
over the entire fleet.
They said improved master cylinder reservoir design, brake fluid and
brake hose materials significantly reduced moisture related problems with
brake fluid. They did however find evidence of corrosion.
This last statement is the key to understanding the new twist being
put on the entire topic of brake fluid testing. Corrosion was found without
significant amounts of moisture in the system. To explain this we have to
take a step back and discuss how one of the purposes of brake fluid is to
prevent corrosion.
 Corrosion inhibitors, pH stabilizers and
antioxidants are added to brake fluid to improve the long-term corrosion
protection of brake systems.
Over time these corrosion inhibitors can become depleted leaving the
internal parts of the brake system vulnerable to corrosion. There are many
variables involved in determining how long it takes to deplete the corrosion
inhibitors including brake fluid chemistry, chemical and thermal stability,
brake system design, driving habits of the operator, frequency of
maintenance, temperature, and road surfaces. Another unrelated extensive
study found that the buffer capacity and inhibitor concentrations "drop to
less than 10% of their initial levels after only 30 months of service".
(Jackson, SAE paper 971007,Corrosion Prevention SP-1265, 1997)
The rate of depletion is affected by many factors. One of the studies
found the rate of depletion is fastest at the wheels. This is where the
fluid is exposed to the highest degree of heat and the heat causes the
corrosion inhibitors to breakdown.
Vehicles with ABS show even faster degradation due to the aggressive
circulation of the fluid caused by the cycling of the ABS system. This,
combined with the fact that ABS systems use close tolerance valves and other
precision parts, makes them more susceptible to the affects of corrosion or
deposits.
Corrosion inhibitors, pH stabilizers and
antioxidants are added to brake fluid to improve the long-term corrosion
protection of brake systems.
Over time these corrosion inhibitors can become depleted leaving the
internal parts of the brake system vulnerable to corrosion. There are many
variables involved in determining how long it takes to deplete the corrosion
inhibitors including brake fluid chemistry, chemical and thermal stability,
brake system design, driving habits of the operator, frequency of
maintenance, temperature, and road surfaces. Another unrelated extensive
study found that the buffer capacity and inhibitor concentrations "drop to
less than 10% of their initial levels after only 30 months of service".
(Jackson, SAE paper 971007,Corrosion Prevention SP-1265, 1997)
The rate of depletion is affected by many factors. One of the studies
found the rate of depletion is fastest at the wheels. This is where the
fluid is exposed to the highest degree of heat and the heat causes the
corrosion inhibitors to breakdown.
Vehicles with ABS show even faster degradation due to the aggressive
circulation of the fluid caused by the cycling of the ABS system. This,
combined with the fact that ABS systems use close tolerance valves and other
precision parts, makes them more susceptible to the affects of corrosion or
deposits.
 One of the task force members submitted a
NHTSA (National Highway Traffic Safety Administration) report that
demonstrates the affect of depleted corrosion inhibitors. In the report they
stated:
"The NIST (National Institute of Standards and Technology) study does
show that internal corrosion does take place as a result of depletion of the
corrosion inhibitors in the brake fluid and the accumulation of water in the
brake fluid over time."
The report went on to offer the following proof of corrosion:
One of the task force members submitted a
NHTSA (National Highway Traffic Safety Administration) report that
demonstrates the affect of depleted corrosion inhibitors. In the report they
stated:
"The NIST (National Institute of Standards and Technology) study does
show that internal corrosion does take place as a result of depletion of the
corrosion inhibitors in the brake fluid and the accumulation of water in the
brake fluid over time."
The report went on to offer the following proof of corrosion:
- Visual evidence of corrosion damage is observed on iron alloy components approximately one-third of the time (typically no damage is observed to stainless steel);
- The damage observed usually consisted of shallow pitting similar to that reported by Jackson's SAE paper;
- In most cases, when corrosion pits were found on iron, copper deposits of varying morphology were also found;
- The small copper particles were found both inside and outside of the shallow pits on the iron, and
- The copper sponge and the copper nugget morphology were found in the shallow pits associated with, and usually under, the gel-like substance.
- interfere with proper ABS valve operation;
- act as an oxidation catalyst;
- provide a precursor warning to active iron corrosion;
- correlate to the age and mileage of vehicular service; and
- correlate to the buffering capability of the brake fluid.
 At this point, you might be asking: "How do
you accurately determine the copper content of the brake fluid?"
The answer comes in the form of test strips that provide a way to
determine the "virtual age" of brake fluid (See Figure 15). The patented
FASCARŪ technology used provides a measure of the copper in the brake fluid
which indirectly provides a measure of the level of corrosion inhibitors in
the system.
The test is simple and straightforward. Simply dip the strip in the
brake fluid of the reservoir for one second. In 30 to 120 seconds, the
reaction zone will change colors depending on the condition of the brake
fluid (See Figure 16). Compare the color of the reaction zone and make the
appropriate recommendation.
Through the hard work of the task force members, the industry has
replaced much of the myth surrounding brake fluid with fact and has adopted
an industry accepted testing technology. Technicians at shops following
these procedures will be able to confidently educate their customers on the
benefits of a brake fluid flush.
Don't be surprised when your customer makes a statement such as:
"Nobody else has ever told me I should change my brake fluid." You will have
to be prepared for this and include the necessary information to educate
your customer as to the need and what you are basing it on.
At this point, you might be asking: "How do
you accurately determine the copper content of the brake fluid?"
The answer comes in the form of test strips that provide a way to
determine the "virtual age" of brake fluid (See Figure 15). The patented
FASCARŪ technology used provides a measure of the copper in the brake fluid
which indirectly provides a measure of the level of corrosion inhibitors in
the system.
The test is simple and straightforward. Simply dip the strip in the
brake fluid of the reservoir for one second. In 30 to 120 seconds, the
reaction zone will change colors depending on the condition of the brake
fluid (See Figure 16). Compare the color of the reaction zone and make the
appropriate recommendation.
Through the hard work of the task force members, the industry has
replaced much of the myth surrounding brake fluid with fact and has adopted
an industry accepted testing technology. Technicians at shops following
these procedures will be able to confidently educate their customers on the
benefits of a brake fluid flush.
Don't be surprised when your customer makes a statement such as:
"Nobody else has ever told me I should change my brake fluid." You will have
to be prepared for this and include the necessary information to educate
your customer as to the need and what you are basing it on.